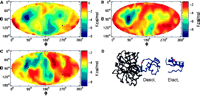
Protein binding dynamics:
Recent advances on our understanding of protein binding
has shown that
the onset of this process is not the fully desolvated interface,
dominated by van der Waals interactions,
but instead a smoother landscape
governed by the the long-range (10 A) electrostatics and intermediate-range
(5 A) solvation forces between molecules.
These forces, which
provide the initial attraction
between ligands and their receptors, were also found
consistent with a relatively narrow docking pathway to
the final bound conformation. On the other hand, it is well known
that surface complementarity, led by short-range vdW forces, play a crucial role
on the stability and specificity of the high affinity complex.
These properties indicate that the intrinsic
dynamic nature of protein binding leads to distinct kinetic regimes
where different driving forces
with diverse length scales governed the binding process at different times.
[1]|[2]|[3]
|